Electrolysis for Green Hydrogen Production
Renewables now provide 30% of global electricity, shifting the conversation from "how do we get clean power?" to "how do we store it?" Green hydrogen is the answer, acting as a chemical battery for the grid. The heavy lifting is done by electrolyzers—machines that use that electricity to split water into fuel. Whether using the rugged, low-cost Alkaline method or high-efficiency steam electrolysis, these four technologies are the practical tools turning a green electricity surplus into a carbon-free industrial reality.
What Is an Electrolyser?
Electrolysers are devices that use electricity to split water into hydrogen and oxygen. Water goes in. Hydrogen and oxygen come out. Everything else inside the system exists to control that process safely and repeatedly.
In green hydrogen setups, the electrolyser is connected to renewable power sources. That connection matters more than the design details. If the electricity is renewable, the hydrogen produced remains low-carbon. If it is not, the environmental benefit quickly disappears. This is why a water electrolyzer for hydrogen production is often treated as part of the energy system rather than a standalone machine.
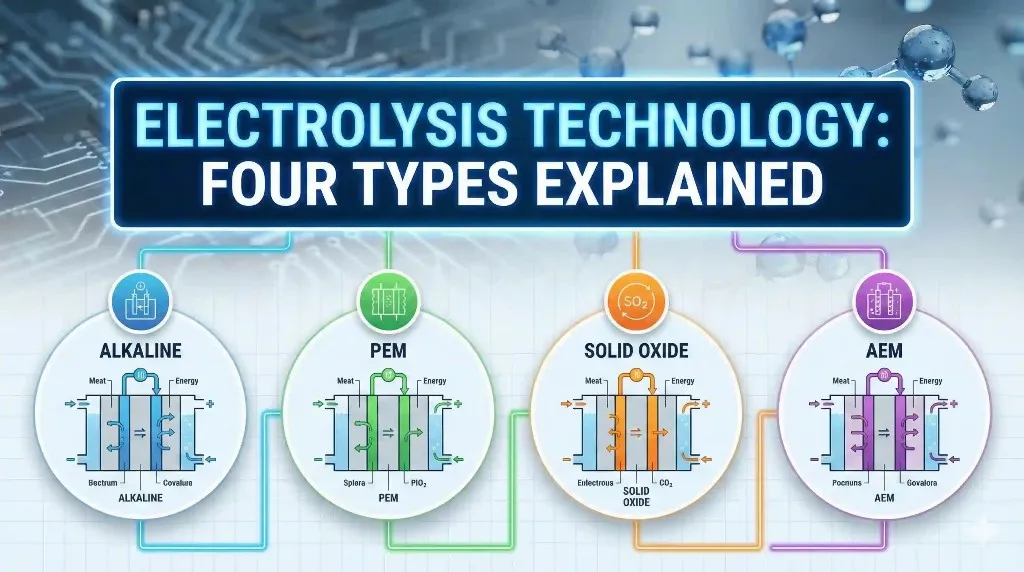
Types of Electrolysers
Different electrolyser designs exist because hydrogen is produced under very different conditions. Some plants run non-stop. Others only operate when renewable power is available.
| Parameters | Alkaline | PEM | AEM | SOEC |
| What it is | The traditional electrolyzer that has been used for decades | A modern electrolyzer that uses a solid polymer membrane | A newer design that mixes ideas from alkaline and PEM systems | A high-temperature electrolyzer that works with steam |
| Electrolyte | Liquid alkaline solution (usually potassium hydroxide) | Solid polymer membrane | Solid membrane that works in alkaline conditions | Solid ceramic material |
| Operating temperature | Around 60–90 °C | Around 50–80 °C | Around 40–70 °C | High-temperature operation: about 650–850 °C |
| Ion that moves | Hydroxide ions (OH⁻) in liquid | Protons (H⁺) through the membrane | Hydroxide ions (OH⁻) through the membrane | Oxygen ions (O²⁻) through ceramic |
| How it works | Electricity splits water in an alkaline liquid; hydrogen forms on one side, oxygen on the other | Water splits at the anode; protons move through the membrane to form hydrogen | Similar to alkaline, but ions move through a solid membrane instead of liquid | Steam is split at high temperature; oxygen moves through the solid electrolyte |
| Catalysts used | Mostly nickel-based, low cost | Precious metals like platinum and iridium | Often non-precious metals | Ceramic and metal-ceramic materials |
| Response to power changes | Slow | Very fast | Fast | Slow (because it stays hot) |
How It Works: A Look Inside the Electrolyzer
A water electrolyzer for hydrogen production operates through a sequence of controlled steps. First, the water is purified and deionized to remove impurities such as salts and chlorine. The purified water is then supplied to the electrolyzer using a controlled pumping system. Inside the electrolyzer, water molecules split into hydrogen and oxygen-related ions, depending on the electrolyte type.
Electrons flow through an external electrical circuit, while charged ions move internally through membranes or liquid electrolytes. Hydrogen forms on one side, oxygen on the other. The membrane electrode assembly ensures that hydrogen and oxygen remain physically separated. The produced gases contain moisture, which is removed using gas-drying systems before hydrogen and oxygen are collected separately.
If pressurized hydrogen is required, compression stages are added downstream of the electrolyzer.
Why Electrolysis-Based Green Hydrogen Matters
Not every energy problem can be solved with a cable and a battery. Heavy industry, chemical feedstocks, and long-duration storage all require something else.
Green hydrogen fits into existing industrial systems with fewer changes than many alternatives. It also offers a way to store renewable electricity when production exceeds demand. In grids with growing solar and wind capacity, this role becomes increasingly important.
Challenges & Considerations in Electrolysis for Green Hydrogen
Electrolysis is expensive when electricity is expensive. That reality dominates every business case.
Upfront costs are still high, especially for systems designed to handle fluctuating power. Some designs depend on specialized materials. Electrolysers use clean water which is again a challenge in water-scarce regions. Even if clean water is available purification adds cost and losses. Beyond production, hydrogen must still be compressed, stored, and moved, which adds cost and complexity.
Conclusion
In short, the shift toward green hydrogen isn't just about the chemistry of water—it’s about finding the best way to "bottle" renewable energy.
The industry has moved past the stage of searching for a single "winner." Instead, we are seeing a toolkit approach:
- Alkaline remains the workhorse for massive, steady industrial plants.
- PEM serves as the flexible bridge for erratic wind and solar power.
- AEM and SOEC represent the next frontier, aiming to slash costs and maximize efficiency through heat integration.
Ultimately, the "best" electrolyser is the one that fits the local environment. Whether an operator prioritizes low upfront costs, rapid response to a shifting power grid, or the use of industrial waste heat, the technology is now mature enough to move out of the lab and into the global energy infrastructure. As renewable electricity becomes the standard rather than the exception, these four technologies will be the primary engines driving a carbon-neutral industrial future.