What Is a Proton Exchange Membrane Fuel Cell
Fuel Cells are electrochemical devices that converts chemical energy into electricity. A PEM Fuel Cell (Proton Exchange Membrane fuel cell), also known as Proton Electrolyte Membrane fuel cell is an electrochemical device that uses a solid polymer membrane (PEM) to generate electricity by combining hydrogen and oxygen. This solid polymer membrane allows protons to pass through while blocking electrons.
Proton Exchange membrane fuel cells are one of the most widely used fuel cells especially for stationary power generation, automotive and academic application due to their low temperature operation.
How Does a PEM Fuel Cell Work
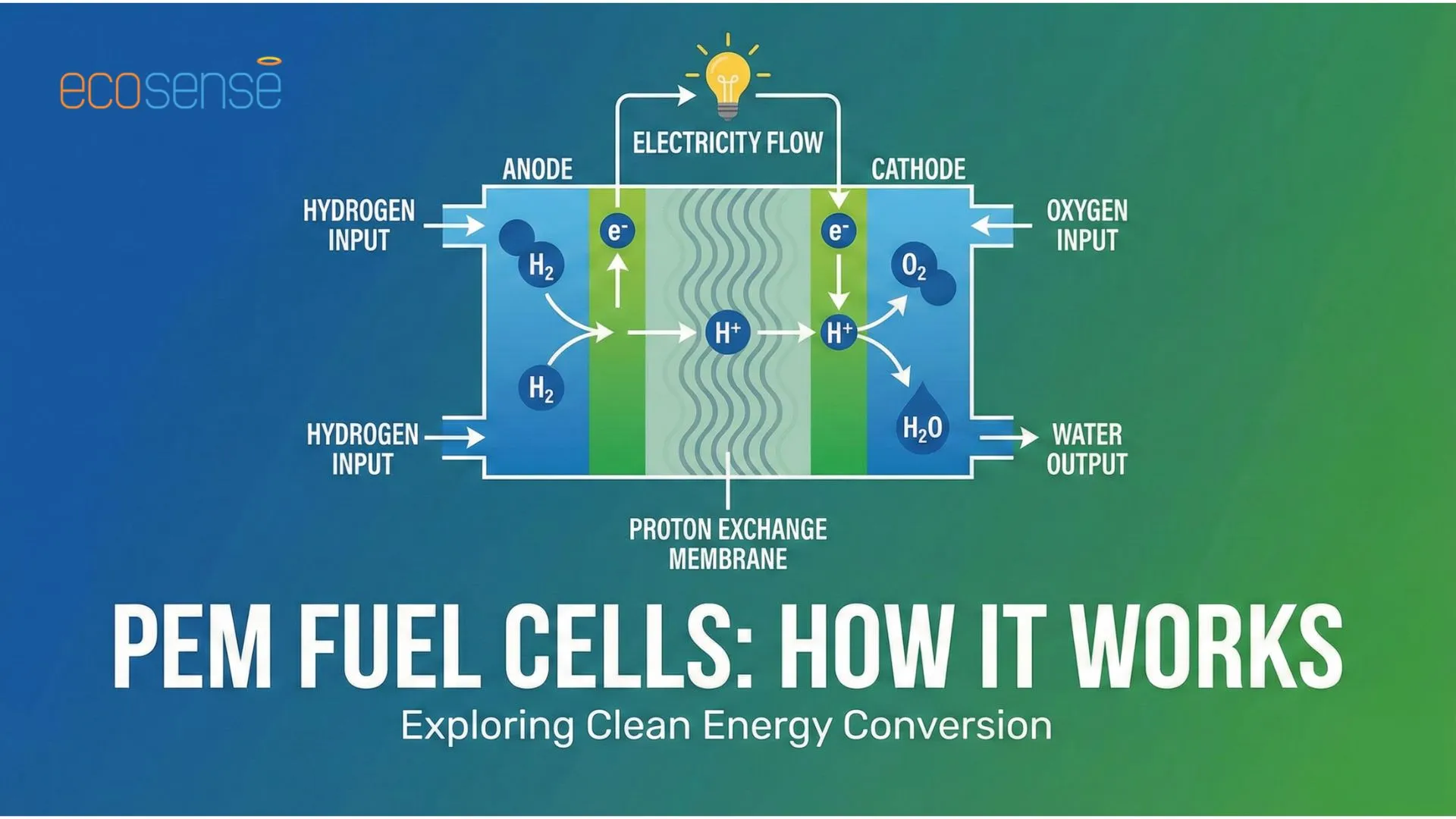
In a PEM fuel cell (Proton Electrolyte Membrane-based fuel cell), hydrogen gas is supplied at the anode and a chemical reaction occurs between hydrogen and a catalyst to produce a hydrogen ion and a free electron. The hydrogen ion diffused through the membrane to the cathode while the electron flows through a circuit to generate current and then combines to form water and heat as the only byproducts.
Overall reaction:
2H₂(g) + O₂(g) → 2H₂O(l)
Anode (Oxidation): Hydrogen gas loses electrons (is oxidized).
H₂(g) → 2H⁺(aq) + 2e⁻ (Acidic Medium)
Cathode (Reduction): Oxygen gas gains electrons (is reduced).
½O₂(g) + 2H⁺(aq) + 2e⁻ → H₂O(l) (Acidic Medium)
The overall process is called a redox reaction where simultaneous oxidation- loss of electrons at the anode and reduction - gain of electrons at the cathode occurs. These reactions are generally slow, so there is a need for catalysts such as platinum to speed up the reaction to give up electrical energy with water and heat as the by-product.
Fig. How PEM Fuel Cell Works
- Role of Anode: Hydrogen is supplied to anode. Anode facilitates hydrogen oxidation and releases electrons to the external circuit as well as supplies protons to the membrane
- Role of Proton Exchange Membrane: The membrane is solid acidic polymer typically made from perfluorosulfonic acid (PFSA) polymers such as Nafion. It only allows protons to pass from anode to cathode and blocks electron forcing them through an external circuit creating current. It also prevents direct mixing of hydrogen and oxygen.
- Role of Cathode: Oxygen is supplied to the cathode. Oxygen reacts with incoming protons and electrons to form water and heat to complete electrochemical circuit.
Key Advantages of PEM Fuel Cells
- High Power Density and Fast Startup: PEM fuel cells can deliver a lot of power from a relatively small system. They start up quickly and respond almost instantly to changes in power demand, which is why they work so well in vehicles and other applications where quick performance matters.
- Low Operating temperature and compact design: Because PEM fuel cells operate at low temperatures, they don’t need long warm-up times or bulky thermal systems. This allows the overall system to stay compact, lightweight, and easier to integrate into real-world and laboratory setups.
- Zero local emission and Quiet Operation: PEM fuel cells generate electricity without combustion, producing only water and heat as by-products. This means no local pollution and very quiet operation, making them ideal for indoor environments, urban mobility, and hands-on learning labs.
Common Uses of PEM Fuel Cell
- PEM fuel cells in vehicle & Mobility: PEM fuel cells are commonly used in cars, buses, and other mobility solutions because they start quickly and deliver smooth, reliable power. Since they emit only water, they are a clean alternative to conventional engines, especially for urban transport. Newly launched Hyundai Nexo is one such example.
- Stationary Power and Backup Application: PEM fuel cells act as dependable backup power sources as they are not weather dependent. They are quiet, efficient, and can run for long durations, making them suitable for telecom towers, hospitals, and data centres.
- Portable Device and Niche Application: PEM fuel cells are also found in portable power units and niche applications such as drones, field equipment, and research systems. Their compact size makes them practical where batteries or diesel generators are not ideal. A few of the companies are making fuel cell powered robots due to the compact nature of PEM Fuel Cells.
Limitations and Challenges of PEM Fuel Cell
- Cost of Material and Platinum Catalyst: One of the main challenges with PEM Fuel Cell is cost. PEM Fuel Cell uses expensive rare earth metals as catalyst especially platinum and iridium which are not easily available and are geographically concentrated within a very limited region. This increases the overall system price and limits large-scale adoption.
- Sensitivity to Fuel impurities and Water Management: Proton Exchange Membrane fuel cells are very sensitive to impurities and generally require 99.996% pure dry hydrogen for their efficient operation. In addition to this water management inside the cell is also critical, too much water can flood the system and too little water can dry out the membrane.
- Durability and Lifetime Issues: Over time, repeated start-stop cycles, temperature changes, and chemical stress can degrade the membrane and catalyst layers. This affects long-term reliability and remains a key area of ongoing research and improvement.
Conclusion
PEM Fuel Cells are wonderful electrochemical devices essential for electricity generation with zero carbon footprint. Their low operating temperature, fast response, and zero local emissions make them especially attractive for modern energy systems. However, challenges related to cost, fuel purity, and long-term durability still limit large-scale deployment. Ongoing advancements in materials, catalyst reduction, and system design are steadily addressing these issues, positioning PEM fuel cells as a key technology in the transition toward a low-carbon and hydrogen-based energy future.